Fig.1
Gunshot Analysis
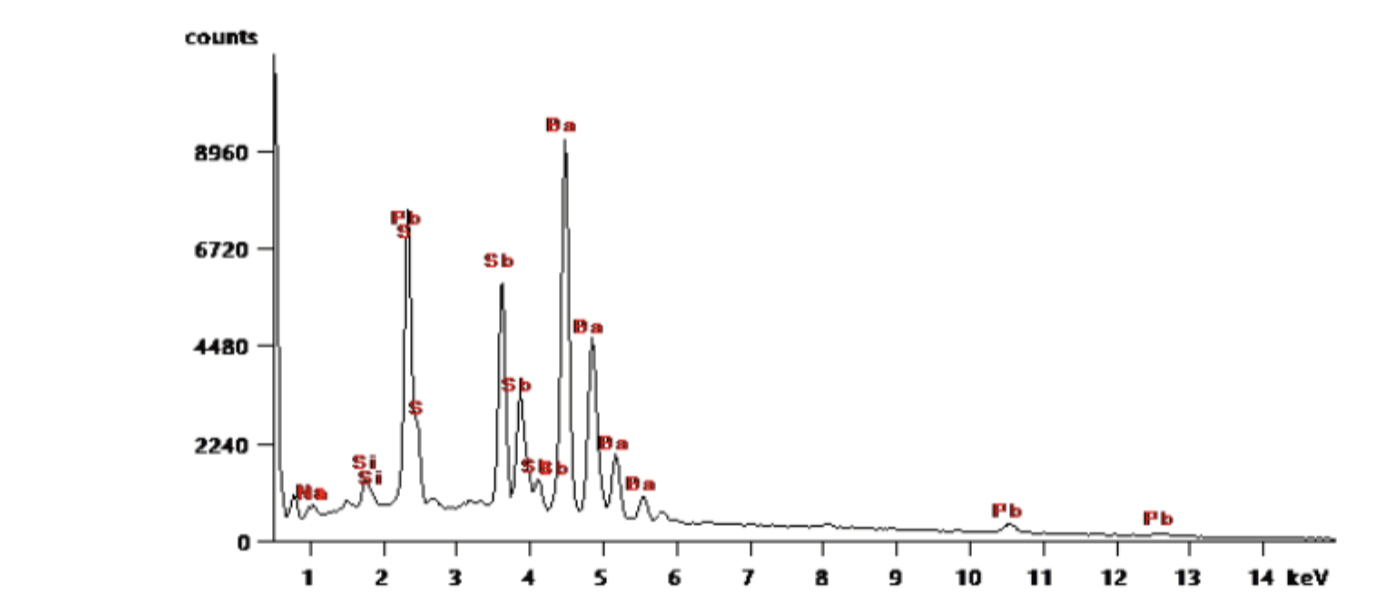
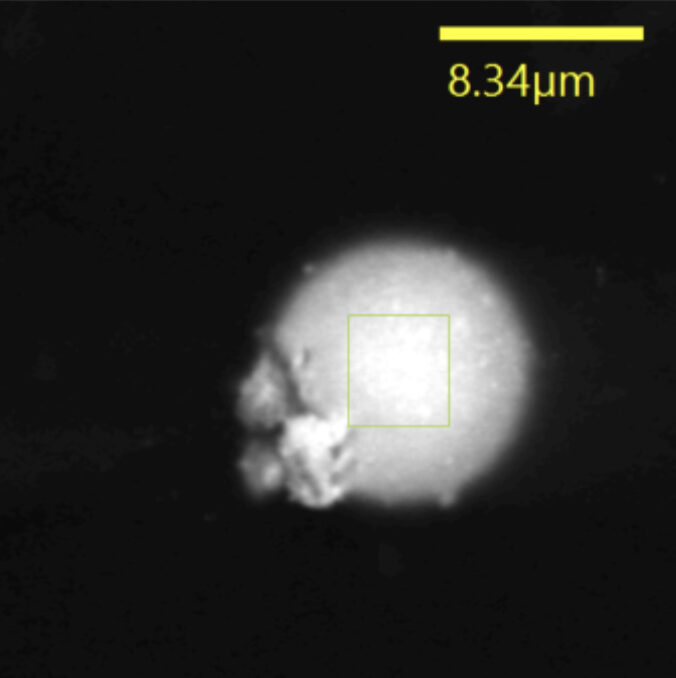
Fig.2
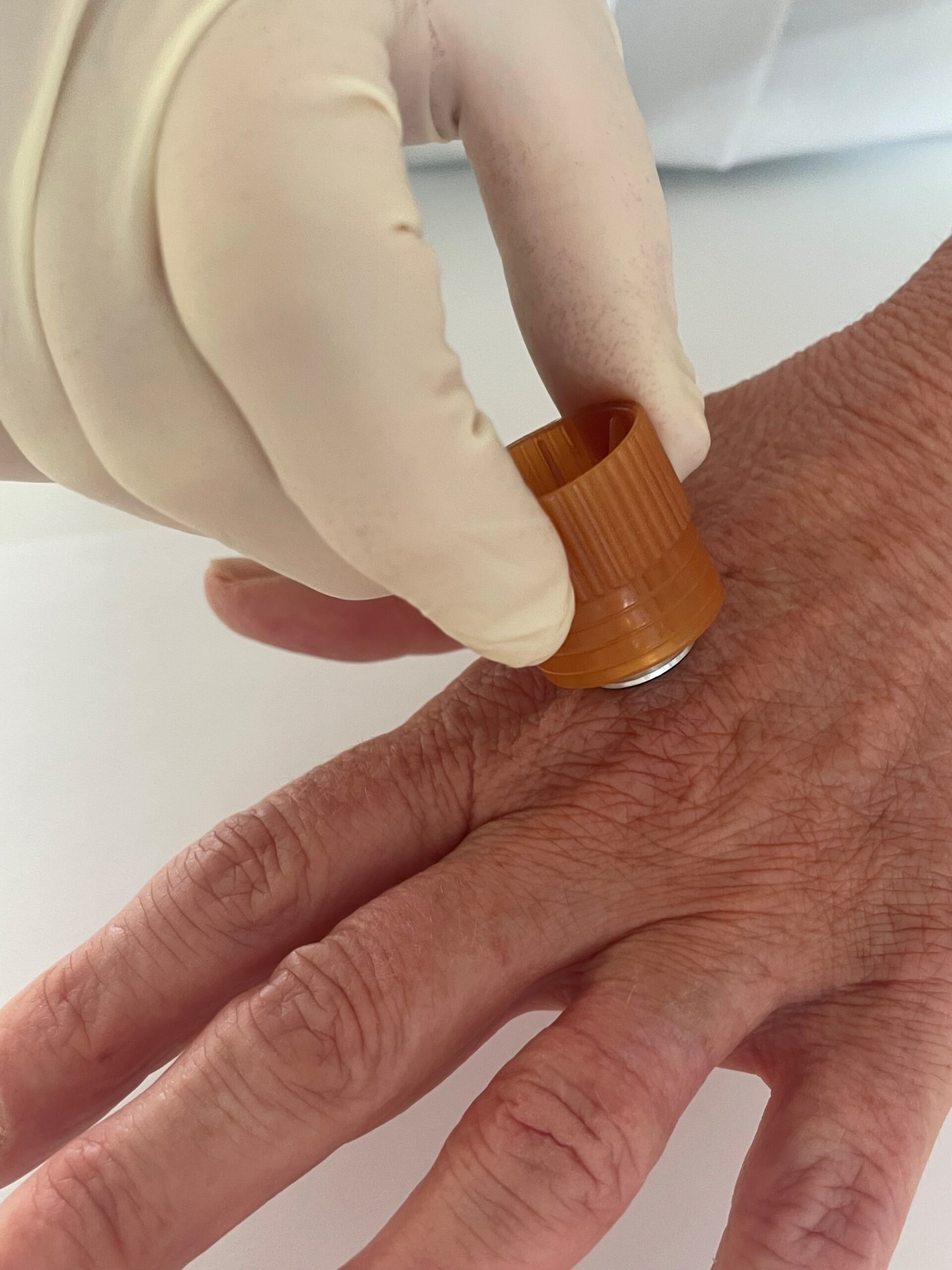
Fig.3
Firearm Residue (Gunshot Residue)
When a firearm is discharged, a cloud of tiny metallic particles is produced by the explosive primer and burning gunpowder. These particles, often invisible to the naked eye, can settle on the hands, clothing, and face of the person who fired the weapon, as well as on nearby surfaces and the firearm itself.
The collection of particles from a gun’s discharge is commonly referred to as gunshot residue (GSR) or sometimes firearm discharge residue (FAR). Some of these microscopic particles have a unique chemical composition that is characteristic of a firearm discharge (typically containing heavy metals from the primer, such as lead, antimony, and barium), while other particles may be consistent with GSR but not unique (since similar particles can come from other sources in the environment). In practice, an examiner looks for a combination of characteristic and consistent particles; if a characteristic mix is present, it strongly indicates the person was in the vicinity of a fired gun. GSR particles transfer at the time of a shooting event and the persistence of GSR is an important factor to consider when sampling.
The analysis of firearm residue is routinely performed using scanning electron microscopy with energy-dispersive X-ray analysis (SEM-EDX). This is a non-destructive technique that provides high-resolution images of the particles along with their elemental composition. Under the electron microscope, true GSR particles appear as roughly spherical and can be chemically identified by the X-ray spectrum (confirming the presence of lead, barium, antimony, etc.). SEM-EDX is considered the international standard for GSR analysis due to its sensitivity and specificity.
